Conductivity: Definition|Equations|Measurements|Applications
Electrical conductivity is far more than an abstract concept; it is the fundamental backbone of our interconnected world, silently powering everything from the latest electronic devices in your hand to the vast power distribution grids that light up our cities.
For engineers, physicists, and materials scientists, or anyone seeking to understand the behavior of matter genuinely, mastering conductivity is non-negotiable. This in-depth guide not only provides a precise definition of conductivity but also unpacks its critical importance, explores the factors influencing it, and highlights its cutting-edge applications across diverse fields like semiconductors, material science, and renewable energy. Just click to explore how understanding this essential property can revolutionize your knowledge of the electrical world.
Table of Contents:
2. Factors Influencing Conductivity
4. How to Measure Conductivity: Equations
5. Tools Used to Measure Conductivity
6. Applications of Conductivity
What is Conductivity?
Electrical conductivity (σ) is a fundamental physical property that quantifies a material’s capacity to support the flow of an electric current. Essentially, it determines how easily charge carriers, primarily free electrons in metals, can traverse a substance. This essential characteristic is the solid basis for countless applications from microprocessors to municipal power infrastructure.
As the reciprocal part of conductivity, the electrical resistivity (ρ) is the opposition to current flow. Therefore, low resistance corresponds directly to high conductivity. The standard international unit for this measurement is Siemens per meter (S/m), though millisiemens per centimeter (mS/cm) is commonly used in chemical and environmental analysis.
Conductivity vs. Resistivity: Conductors vs. Insulators
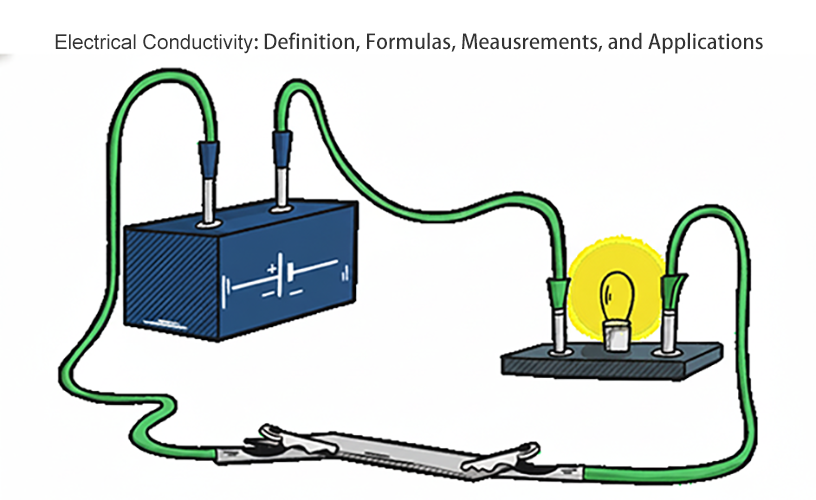
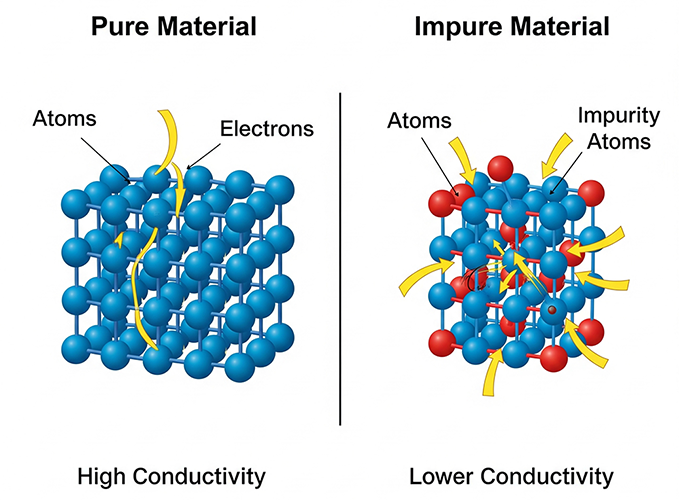
Exceptional conductivity (σ) designates materials as conductors, while pronounced resistivity (ρ) renders them ideal insulators. Fundamentally, the stark contrast in material conductivity originates from the differential availability of mobile charge carriers.
High Conductivity (Conductors)
Metals like copper and aluminum exhibit extremely high conductivity. This is due to their atomic structure, which features a vast ‘sea’ of easily movable valence electrons that are not strongly bound to individual atoms. This property makes them indispensable for electrical wiring, power transmission lines, and high-frequency circuit traces.
If you are eager to know more material’s conduction of electricity, feel free to read the post focusing on revealing the electricity conductivity of all the materials in your life.
Low Conductivity (Insulators)
Materials such as rubber, glass, and ceramics are known as insulators. They possess few to no free electrons, strongly resisting the passage of electric current. This characteristic makes them vital for safety, isolation, and preventing short circuits in all electrical systems.
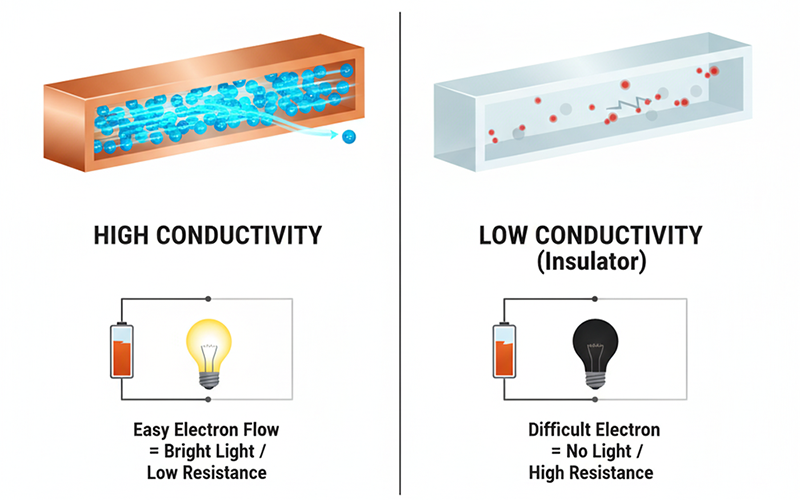
Factors Influencing Conductivity
Electrical conductivity is a foundational material property, but contrary to a common misconception, it is not a fixed constant. The ability of a material to conduct an electrical current can be profoundly and predictably influenced by external environmental variables and precise compositional engineering. Understanding these factors is the bedrock of modern electronics, sensing, and energy technologies:
1. How External Factors Influence Conductivity
The material’s immediate environment exerts significant control over the mobility of its charge carriers (typically electrons or holes). Let’s explore them in detail:
1. Thermal Effects: The Impact of Temperature
Temperature is perhaps the most universal modifier of electrical resistance and conductivity.
For the vast majority of pure metals, conductivity decreases as temperature rises. The thermal energy causes the metal’s atoms (the crystal lattice) to vibrate with greater amplitude, and consequently, these intensified lattice vibrations (or phonons) increase the frequency of scattering events, effectively impeding the smooth flow of valence electrons. This phenomenon explains why overheated wires lead to power loss.
Conversely, in semiconductors and insulators, conductivity dramatically increases with rising temperature. The added thermal energy excites electrons from the valence band across the band gap and into the conduction band, thus creating a greater number of mobile charge carriers and significantly lowering resistivity.
2. Mechanical Stress: The Role of Pressure and Strain
Applying mechanical pressure can alter the atomic spacing and crystal structure of a material, which in turn influences conductivity, and this is a phenomenon critical in piezoresistive sensors.
In some materials, compressive pressure forces atoms closer together, enhancing the overlap of electron orbitals and making the movement of charge carriers easier, thereby increasing conductivity.
In materials like silicon, stretching (tensile strain) or squeezing (compressive strain) can rearrange the electron energy bands, altering the effective mass and mobility of the charge carriers. This precise effect is leveraged in strain gauges and pressure transducers.
2. How Impurity Influences Conductivity
In the realm of solid-state physics and microelectronics, the ultimate control over electrical properties is achieved through compositional engineering, primarily via doping.
Doping is the highly controlled introduction of trace amounts of specific impurity atoms (typically measured in parts per million) into a highly purified, intrinsic base material, such as silicon or germanium.
This process does not just change conductivity; it fundamentally tailors the material’s carrier type and concentration to create predictable, asymmetrical electrical behavior necessary for computing:
N-Type Doping (Negative)
Introducing an element with more valence electrons (e.g., Phosphorus or Arsenic, which have 5) than the host material (e.g., Silicon, which has 4). The extra electron is easily donated to the conduction band, making the electron the primary charge carrier.
P-Type Doping (Positive)
Introducing an element with fewer valence electrons (e.g., Boron or Gallium, which have 3). This creates an electron vacancy, or ‘hole,’ which acts as a positive charge carrier.
The ability to precisely control conductivity through doping is the engine of the digital age:
For semiconductor devices, it is used to form p-n junctions, the active regions of diodes and transistors, which allow current flow in only one direction and serve as the core switching elements in Integrated Circuits (ICs).
For thermoelectric devices, conductivity control is crucial for balancing the need for good electrical conduction (to move charge) against poor thermal conduction (to maintain a temperature gradient) in materials used for power generation and cooling.
From the perspective of advanced sensing, materials can be doped or chemically modified to create chemiresistors, whose conductivity changes dramatically upon binding to specific gases or molecules, forming the basis of highly sensitive chemical sensors.
Understanding and precisely controlling conductivity remains critical for developing next-generation technologies, ensuring optimal performance, and maximizing efficiency across virtually every sector of science and engineering.
Conductivity Units
The standard SI unit for conductivity is Siemens per meter (S/m). However, in most industrial and laboratory settings, Siemens per centimeter (S/cm) is the more common base unit. Because conductivity values can span many orders of magnitude, measurements are typically expressed using prefixes:
1. microSiemens per centimeter (mS/cm) is used for low-conductivity liquids like deionized or reverse osmosis (RO) water.
2. milliSiemens per centimeter (mS/cm) is common for tap water, process water, or brackish solutions (1 mS/cm = 1,000 μS/cm).
3. deciSiemens per meter (dS/m) is often used in agriculture and is equivalent to mS/cm (1 dS/m = 1 mS/cm).
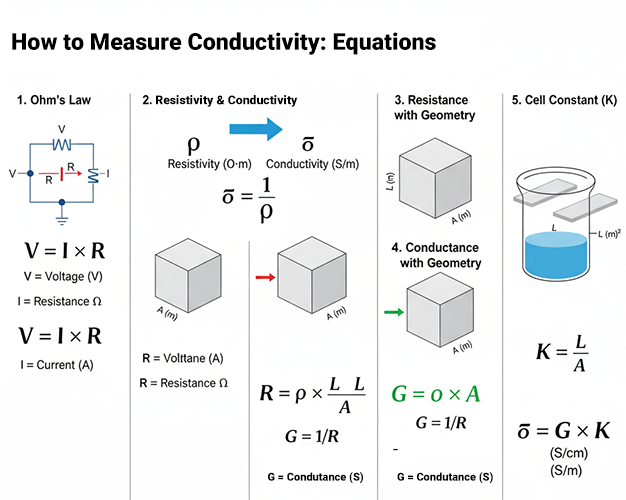
How to Measure Conductivity: Equations
A conductivity meter does not measure conductivity directly. Instead, it measures conductance (in Siemens) and then calculates conductivity using a sensor-specific Cell Constant (K). This constant (with units of cm -1) is a physical property of the sensor’s geometry. The instrument’s core calculation is:
Conductivity (S/cm) = Measured Conductance (S) × Cell Constant (K, in cm⁻¹)
The method used to obtain this measurement depends on the application. The most common method involves contacting (Potentiometric) sensors, which use electrodes (often graphite or stainless steel) that are in direct contact with the liquid. A simple 2-electrode design is effective for low-conductivity applications like pure water. More advanced 4-electrode sensors provide high accuracy across a much broader range and are less susceptible to errors from moderate electrode fouling.
For harsh, corrosive, or highly conductive solutions where electrodes would foul or corrode, inductive (Toroidal) sensors come into play. These non-contact sensors feature two wire-wound coils encapsulated in a durable polymer. One coil induces an electrical current loop in the solution, and the second coil measures the magnitude of this current, which is directly proportional to the liquid’s conductivity. This design is extremely robust as no metal parts are exposed to the process.
Measurements of Conductivity and Temperature
Conductivity measurements are highly dependent on temperature. As a liquid’s temperature increases, its ions become more mobile, causing the measured conductivity to rise (often by ~2% per °C). To ensure measurements are accurate and comparable, they must be normalized to a standard reference temperature, which is universally 25°C.
Modern conductivity meters perform this correction automatically using an integrated temperature sensor. This process, known as Automatic Temperature Compensation (ATC), applies a correction algorithm (such as the linear formula G25= G_t/[1+α(T-25)]) to report the conductivity as if it were measured at 25°C.
Where:
G₂₅ = Corrected Conductivity at 25°C;
G_t = Raw conductivity measured at the process temperature T;
T = The measured process temperature (in °C);
α (alpha) = The temperature coefficient of the solution (e.g., 0.0191 or 1.91%/°C for NaCl solutions).
Measure Conductivity with Ohm’s Law
Ohm’s Law, a cornerstone of electrical science, provides a practical framework for quantifying a material’s electrical conductivity (σ). This principle establishes the direct correlation between voltage (V), current (I), and resistance (R). By extending this law to include a material’s physical geometry, its intrinsic conductivity can be derived.
The first step is to apply Ohm’s Law (R = V/I) to a specific material sample. This requires taking two precise measurements: the voltage applied across the sample and the current that flows through it as a result. The ratio of these two values yields the sample’s total electrical resistance. This calculated resistance, however, is specific to that sample’s size and shape. To normalize this value and determine the material’s inherent conductivity, one must account for its physical dimensions.
The two critical geometric factors are the sample’s length (L) and its cross-sectional area (A). These elements are integrated into a single formula: σ = L / (R^A).
This equation effectively translates the measurable, extrinsic property of resistance into the fundamental, intrinsic property of conductivity. It is critical to recognize that the final calculation’s accuracy is directly dependent on the quality of the initial data. Any experimental errors in measuring V, I, L, or A will compromise the validity of the computed conductivity.
Tools Used to Measure Conductivity
In industrial process control, water treatment, and chemical manufacturing, electrical conductivity is not just a passive measurement; it’s a critical control parameter. Achieving accurate, repeatable data doesn’t come from a single, all-purpose tool. Instead, it requires building a complete, matched system where each component is chosen for a specific task.
A robust conductivity system consists of two primary parts: the controller (the brain) and the sensor (the senses), both of which must be supported by proper calibration and compensation.
1. The Core: The Conductivity Controller
The central hub of the system is the online conductivity controller, which does far more than just display a value. This controller acts as the “brain,” powering the sensor, processing the raw signal, and making the data useful. Its key functions include the following:
① Automatic Temperature Compensation (ATC)
Conductivity is highly sensitive to temperature. An industrial controller, like the SUP-TDS210-B or the high-precision SUP-EC8.0, uses an integrated temperature element to automatically correct every reading back to the 25°C standard. This is essential for accuracy.
② Outputs and Alarms
These units translate the measurement into a 4-20mA signal for a PLC, or trigger relays for alarms and dosing pump control.
③ Calibration Interface
The controller is configured with a software interface to perform regular, simple calibrations.
2. Selecting the Right Sensor
The most critical section is the choice you make regarding the sensor (or probe), as its technology must match the properties of your liquid. Using the wrong sensor is the number one cause of measurement failure.
For Pure Water & RO Systems (Low Conductivity)
For applications such as reverse osmosis, deionized water, or boiler feedwater, the liquid contains very few ions. Here, a two-electrode conductivity sensor (like the SUP-TDS7001) is the ideal choice to measure the conductivity of water. Its design provides high sensitivity and accuracy at these low conductivity levels.
For General Purpose & Wastewater (Mid-to-High Conductivity)
In dirty solutions, containing suspended solids or having a wide measurement range (like wastewater, tap water, or environmental monitoring), sensors are prone to fouling. In such a case, a four-electrode conductivity sensor like the SUP-TDS7002 is the superior solution. This design is less affected by buildup on the electrode surfaces, offering a much wider, more stable, and more reliable reading in variable conditions.
For Harsh Chemicals & Slurries (Aggressive & High Conductivity)
When measuring aggressive media, such as acids, bases, or abrasive slurries, traditional metal electrodes will corrode and fail quickly. The solution is a non-contact inductive (toroidal) conductivity sensor like the SUP-TDS6012 lineup. This sensor uses two encapsulated coils to induce and measure a current in the liquid without any part of the sensor touching it. This makes it virtually immune to corrosion, fouling, and wear.
3. The Process: Ensuring Long-Term Accuracy
The system’s reliability is maintained through one critical process: calibration. A controller and sensor, no matter how advanced, must be checked against a known reference solution (a conductivity standard) to ensure accuracy. This process compensates for any minor sensor drift or fouling over time. A good controller, like the SUP-TDS210-C, makes this a simple, menu-driven procedure.
Achieving precise conductivity measurement is a matter of smart system design. It requires matching an intelligent controller with a sensor technology built for your specific application.
What is the best material for conducting electricity?
The best material for conducting electricity is pure silver (Ag), boasting the highest electrical conductivity of any element. However, its high cost and tendency to tarnish (oxidize) limit its widespread application. For most practical uses, copper (Cu) is the standard, as it offers the second-best conductivity at a much lower cost and is highly ductile, making it ideal for wiring, motors, and transformers.
Conversely, gold (Au), despite being less conductive than both silver and copper, is vital in electronics for sensitive, low-voltage contacts because it possesses superior corrosion resistance (chemical inertness), which prevents signal degradation over time.
Finally, aluminum (Al) is utilized for long-distance, high-voltage transmission lines because its lighter weight and lower cost offer significant advantages, despite its lower conductivity by volume compared to copper.
Applications of Conductivity
As a material’s intrinsic ability to transmit electrical current, electrical conductivity is a fundamental property that drives technology. Its application spans everything from large-scale power infrastructure to micro-scale electronics and environmental monitoring. Below are its key applications where this property is essential:
Power, Electronics, and Manufacturing
High conductivity is the bedrock of our electrical world, while controlled conductivity is crucial for industrial processes.
Power Transmission and Wiring
High-conductivity materials like copper and aluminum are the standard for electrical wiring and long-distance power lines. Their low resistance minimizes I2R (Joule) heating losses, ensuring efficient energy transmission.
Electronics and Semiconductors
At a micro level, conductive traces on Printed Circuit Boards (PCBs) and connectors form the pathways for signals. In semiconductors, the conductivity of silicon is precisely manipulated (doped) to create transistors, the basis of all modern integrated circuits.
Electrochemistry
This field relies on the ionic conductivity of electrolytes. This principle is the engine for batteries, fuel cells, and industrial processes like electroplating, metal refining, and the production of chlorine.
Composite Materials
Conductive fillers (like carbon or metal fibers) are added to polymers to create composites with specific electrical properties. These are used for electromagnetic shielding (EMI) to protect sensitive devices and for electrostatic discharge (ESD) protection in manufacturing.
Monitoring, Measurement, and Diagnostics
The measurement of conductivity is as critical as the property itself, serving as a powerful analytical tool.
Water Quality and Environmental Monitoring
Conductivity measurement is a primary method for assessing water purity and salinity. Since dissolved ionic solids (TDS) directly increase conductivity, sensors are used to monitor drinking water, manage wastewater treatment, and assess soil health in agriculture.
Medical Diagnostics
The human body functions on bioelectrical signals. Medical technologies like Electrocardiography (ECG) and Electroencephalography (EEG) work by measuring the minute electrical currents conducted by ions in the body, allowing for the diagnosis of cardiac and neurological conditions.
Process Control Sensors
In chemical and food manufacturing, conductivity sensors are used to monitor processes in real-time. They can detect changes in concentration, identify interfaces between different liquids (e.g., in clean-in-place systems), or warn of impurities and contamination.
FAQs
Q1: What is the difference between conductivity and resistivity?
A: Conductivity (σ) is a material’s ability to permit electric current, measured in Siemens per meter (S/m). Resistivity (ρ) is its ability to oppose current, measured in Ohm-meters (Ω⋅m). They are direct mathematical reciprocals (σ=1/ρ).
Q2: Why do metals have high conductivity?
A: Metals use metallic bonding, where valence electrons are not bound to any single atom. This forms a delocalized “sea of electrons” that moves freely through the material, easily creating a current when a voltage is applied.
Q3: Can conductivity be changed?
A: Yes, conductivity is highly sensitive to external conditions. The most common factors are temperature (rising temperatures decrease conductivity in metals but increase it in water) and the presence of impurities (which disrupt electron flow in metals or add ions to water).
Q4: What makes materials like rubber and glass good insulators?
A: These materials have strong covalent or ionic bonds where all valence electrons are tightly held. With no free electrons to move, they cannot support an electrical current. This is known as having a very large “energy band gap.”
Q5: How is conductivity measured in water?
A: A meter measures ionic conductivity from dissolved salts. Its probe applies an AC voltage to the water, causing dissolved ions (like Na+ or Cl−) to move and create a current. The meter measures this current, automatically corrects for temperature, and uses the sensor’s “cell constant” to report the final value (usually in μS/cm).
Post time: Oct-24-2025